Abstract
Mutations in the erythropoietin-receptor gene (EPOR) are well known causes of inherited forms of primary erythrocytosis that is characterized by low serum erythropoietin (Epo) levels. Secondary erythrocytosis with elevated Epo serum levels is mainly caused by mutations in genes involved in oxygen sensing pathway. Epo is the primary regulator of erythropoiesis, but mutations in the EPO gene have so far not been described as a cause or erythrocytosis. Here we studied a Norwegian family with autosomal dominant erythrocytosis and elevated Epo serum levels.
We performed genome-wide linkage analysis using SNP arrays on 16 informative family members including 10 affected individuals and identified a co-segregating region on chromosome 7q22.1 with a LOD score of 3.3. Targeted sequencing of all 215 genes within the co-segregating region revealed only a heterozygous single base deletion in exon 2 of EPO as the sole candidate gene mutation. ThisΔG deletion creates a frame-shift that truncates the Epo signal peptide and generates a novel peptide, terminating after additional 51 amino acids. Thus, contrary to the expectation based on the erythrocytosis phenotype, the EPO ΔG deletion was predicted to be a loss-of-function mutation.
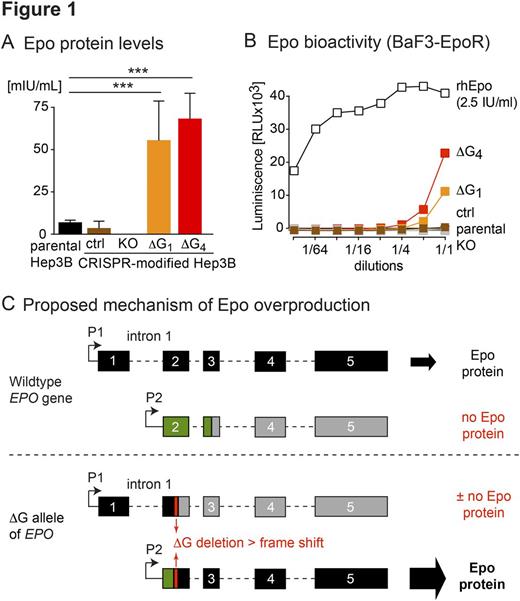
Since we had no access to patient's kidney or liver tissues where Epo is produced, we used the CRISPR technique to introduce the ΔG mutation into the EPO gene into Hep3B cells, a human hepatoma cell line that expresses EPO . We obtained two Hep3B single-cell derived clones (ΔG1 and ΔG4) that were homozygous for the EPO ΔG mutation and measured Epo protein in the culture supernatants. We found that these supernatants contained 8-10 times more Epo than parental Hep3B cells or controls, as determined by ELISA (Figure 1A), and were also capable of stimulating the growth of an Epo-dependent cell line (BaF3- EpoR), demonstrating biological activity (Figure 1B). Thus, the ΔG deletion introduced into the EPO gene locus in Hep3B cells behaved as a gain-of-function mutation.
To decipher the mechanism of how the ΔG mutation was able to produce the excess of Epo protein, we searched for alternative EPO mRNAs by 5'-RACE. In addition to the expected transcripts originating from the physiologic promoter (P1) upstream of exon 1, we discovered two types of alternative mRNAs that initiate in intron 1 of EPO. Both initiatied from a putative alternative promoter (P2) in intron 1 that contains two GATA motifs. In addition to Hep3B ΔG clones, P2 transcripts were also detected in parental Hep3B cells and also in mRNA from normal kidney and liver.
To determine which transcripts are capable of producing Epo, we transfected HEK293 cells with cDNAs representing P1 or P2 transcripts with or without the ΔG mutation. Supernatants of cells transfected with P2 ΔG transcripts contained more Epo than cells transfected with the wildtype P1 cDNA. These supernatants also stimulated the growth of Epo dependent BaF3- EpoR cells and supported erythroid colony formation of progenitors from human peripheral blood, demonstrating that P2 ΔG transcripts produce biologically active Epo protein.
Analysis of the P2 mRNA sequences shows that the normally non-coding P2 transcripts through the ΔG single base deletion connect to the Epo coding sequence creating a functional signal peptide with a novel N-terminus, but retaining the physiologic Epo signal peptide cleavage site. These P2 ΔG transcripts are more abundant in liver and produce an excess of biologically active Epo by escaping the oxygen sensing regulation. Our data demonstrate for the first time mutation in EPO as a cause of familial erythrocytosis and provide an explanation of how the ΔG results in a gain-of-function mutation (Figure 1C).
Figure 1 legend: A) Production of Epo protein by He3B cells carrying the ΔG mutation. Supernatants of parental Hep3B or CRISPR-modified Hep3B clones were collected and Epo protein was measured by Epo ELISA. The levels of Epo are given as a mean ± SD (n=3), p < 0.001 = *** as determined by one-way ANOVA. B) Biological activity of Epo produced by parental Hep3B or CRISPR-modified Hep3B cells measured by a proliferation assay with BaF3-EpoR cell line. BaF3-EpoR cells were grown in serial dilutions of supernatants and cell numbers were assessed by Cell Titer-Glo Assay, (n=3). Serial dilutions of 2.5 IU/ml of recombinant human Epo were used for reference. C) Model and proposed mechanism for EPO overproduction by a single nucleotide deletion in exon 2 of the EPO gene.
Waage: Celgene, Takeda: Honoraria. Skoda: Novartis: Consultancy, Speakers Bureau; Shire: Speakers Bureau; Baxalta: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal